Abstract
Background: Relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (B-ALL) is an aggressive malignant disease with poor long-term outcomes. Blinatumomab, a bispecific T-cell engager (BiTe) CD3-CD19 antibody, showed improved overall survival compared to standard chemotherapy. This is a long-term follow-up analysis evaluating outcomes of adult patients with R/R B-ALL treated in a phase 2 trial of blinatumomab at a single institution.
Methods: Adult patients with R/R Philadelphia negative B-ALL were enrolled in this open-label single arm phase 2 trial from January 2012 until January 2015. During screening period, patients who had high tumor burden were given 10 mg/m2/day of dexamethasone for up to 5 days until 3 days prior to treatment initiation in order to prevent cytokine release syndrome. Patients received continuous IV infusion of blinatumomab 28 µg/day over 4 weeks (wk) followed by 2 wk treatment-free interval. For the first cycle, blinatumomab was initiated at 9 µg/day for 1 wk and then escalated to 28 µg/day, if tolerated. Patients who achieved response within 2 cycles received up to 3 additional cycles of consolidation treatment (total of 5 cycles). Allogeneic stem cell transplantation (ASCT) was offered to responding patients at the discretion of the investigators. The primary objective of the trial was to evaluate efficacy as well as safety of blinatumomab in patients with R/R B-ALL. During long-term follow-up, hematological relapse, treatment received after blinatumomab failure and survival data were collected. Overall survival was defined as the time elapsed from the date of start of treatment until death or date of last follow-up.
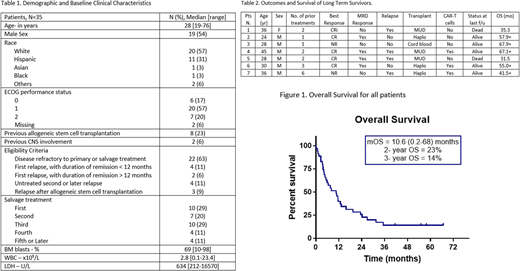
Results: A total of 35 patients received blinatumomab, with baseline characteristics summarized in Table 1. The median number of cycles received was 2 (range, 1-5 cycles). The overall response rate was 54% (19/35), with only 2 achieving response after 2 cycles. The median time to response was 28 days (range, 14-69 days). Complete remission (CR), CR with partial hematologic recovery (CRp), CR with incomplete hematologic recovery (CRi) were achieved in 13 (37%), 4 (11%), and 2 (5%) patients, respectively. Sixteen out of 19 (84%) responding patients achieved negative minimal residual disease (MRD) by flow cytometry. Treatment was well tolerated with most adverse events being transient. Cytokine release syndrome was observed in 17 (49%) patients, all were of grade 2 toxicity. Neurotoxicity of grade 1 and 2 were observed in 9 (26%) patients; 5 patients had tremors, 3 had confusion, and 1 had seizure. Of all patients, 17 (49%) underwent subsequent ASCT: 6 (32%) had ASCT as consolidation after blinatumomab; of them 3 remained in continuous MRD negativity post ASCT for a median of 53.6 months (range, 2.4-57). Of the 19 responding patients, 16 relapsed after a median of 3 months (range, 0.5 to 26.1); 4 of them (25%) with CD19-negative disease. Eleven patients received subsequent ASCT after other salvage strategies. Anti-CD19 therapies were given to 8 (23%) patients after failure. Two patients received an anti-CD19 drug conjugate, and 6 other patients received anti-CD19 CAR T cells (2 patients for relapsed disease after ASCT and 4 patients as consolidation post-ASCT). None of the patients was re-challenged with blinatumomab after relapse. After a median follow-up of 11 months, 5 patients (14%) remained alive. The median OS was 10.6 months (range, 0.2-68). The 2-year and 3-year OS were 23% and 14% respectively. Outcomes of long-term survivors (i.e. >2 years) are summarized in Table 2.
Conclusion: Blinatumomab followed by ASCT confers good long-term survival among patients with heavily pretreated R/R ALL. Long-term survival of more than 2 years was only observed in those who received subsequent ASCT.
Short:Takeda Oncology: Consultancy. Ravandi:Macrogenix: Honoraria, Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Abbvie: Research Funding; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Xencor: Research Funding; Sunesis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Orsenix: Honoraria; Xencor: Research Funding; Sunesis: Honoraria; Jazz: Honoraria; Jazz: Honoraria. Sasaki:Otsuka Pharmaceutical: Honoraria. Cortes:Astellas Pharma: Consultancy, Research Funding; Arog: Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding. O'Brien:Pfizer: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Alexion: Consultancy; Regeneron: Research Funding; TG Therapeutics: Consultancy, Research Funding; Astellas: Consultancy; Celgene: Consultancy; Aptose Biosciences Inc.: Consultancy; Janssen: Consultancy; Kite Pharma: Research Funding; GlaxoSmithKline: Consultancy; Sunesis: Consultancy, Research Funding; Abbvie: Consultancy; Pharmacyclics: Consultancy, Research Funding; Vaniam Group LLC: Consultancy; Acerta: Research Funding; Amgen: Consultancy. Jabbour:Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Abbvie: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.